Blog
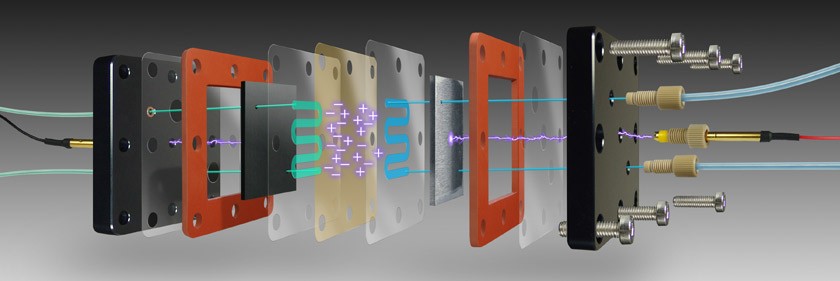
Flow Electrolysis
Published: August 10, 2022Flow electrolysis is a method of production which boasts vast amounts of potential in chemical research. As many recent works show, there is a growing need for flow electrolysis cells and the research advantages that these devices provide. As many researchers have discovered, flow electrolysis cells provide a boost to a wide variety of research activities and should be in the arsenal of any chemist looking to increase their throughput while maintaining environmental consciousness. As a trusted name in the life sciences industry, Analytical Sales and Services is ready to be your partner in this chemistry. A few articles detailing the advantages of flow electrolysis follow below.
Flow electrolysis has been used in research to produce products in a “green” manner by requiring less electrolyte. Using a lab-constructed electrolysis flow cell, Horii et. al. were able to perform methoxylation and acetoxylation of furan, without needing any supporting electrolyte at all. By varying the flowrate, electrode material, and current density, the researchers were able to achieve Furan conversions beyond 99%. The methoxylation also exhibited high selectivity, with yields of 2,5-dimethoxy-2,5-dihydrofuran also exceeding 99%. (1)
Wang et. al. studied the oxidation of alcohols via electrolysis using a continuous-flow reactor. While previous researchers were able to perform direct electro-oxidation with a non-continuous undivided flow cell, their yield and selectivity were low. Wang et. al. proposed and executed an experiment in which the desired aldehydes and ketones were produced using a continuous flow setup, allowing for the avoidance of environmentally unfriendly catalysts such as chromium or ruthenium salts. Once the proper experimental conditions had been found, it was observed that the reactions were able to produce yields of certain aldehydes as high as 99%. (2)
Cusick et. al. studied the performance of scaled up microbial flow electrolysis cells. These devices were shown to enable the conversion of organic waste into useful chemical species such as methane or hydrogen. The research performed by Cusick et. al. was the first instance of a microbial electrolysis cell being made to operate within the thousand liter scale while being fed actual wastewater. Using the flow electrolysis setup that they designed, it was found that the flow cell reactor was able to produce enough biogas to have powered the setup with energy to spare. This reactor represents an important step in the pursuit of renewable energy. (3)
Hardwick et. al. used flow electrolysis to preserve the chirality of compounds. Attempting to generate many medicinally useful compounds may lead to racemic mixtures even when starting from enantiopure compounds. Since typically only a particular product enantiomer will function as desired, this necessitates asymmetric synthesis. By varying the current of their electrolytic setup, the researchers were able to control the memory of chirality and vary the enantiomeric excess of the product. Even when the experiment was performed at a temperature disadvantage, the researchers were still able to confirm that the performance obtained from flow electrolysis was superior to that of a batch reactor. (4)
The benzothiazole scaffold is an important constituent of many bioactive compounds. Traditionally, these are produced from N-arylthioamides using iron-based catalysts. As these catalysts would need to be removed from the product material, it was desired to find a way to obviate their use while also avoiding the transformation of thioamides into amides. Folguerias-Amador and others were able to synthesize benzothiazoles and thiazolopyridines by using a continuous flow electrolysis setup without catalysts or electrolytes. This allows for greater reactant efficiency while still providing the increased output that is typical of flow electrolysis reactors. The researchers were able to perform reactions that saw conversions greater than 99%. These reactions were able to be performed outside of an inert atmosphere and their production on the gram-scale served as further conformation of the scalability of these processes. (5)
For many researchers, flow electrolysis is proving to be a source of greatly improved efficiency and yield, while also providing opportunities for environmentally friendly chemical production by removing the need for many additives that may prove toxic or expensive. As a continuous method, flow electrolysis allows for a confirmation of scalability. If you would like to be at the forefront of this new wave of research with a partner that you can trust, allow Analytical Sales and Services to support you with trusted results.
References
- Daisuke Horii et al 2006 J. Electrochem. Soc. 153 D143
- Wang, D., Wang, P., Wang, S. et al. Direct electrochemical oxidation of alcohols with hydrogen evolution in continuous-flow reactor. Nat Commun 10, 2796 (2019)
- Cusick, R.D., Bryan, B., Parker, D.S. et al. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl Microbiol Biotechnol 89, 2053–2063 (2011)
- Hardwick, T., Cicala, R., Wirth, T. et al. Memory of chirality in a room temperature flow electrochemical reactor. Sci Rep 10, 16627 (2020)
- A. A. Folgueiras-Amador, X.-Y. Qian, H.-C. Xu, T. Wirth, Chem. Eur. J. 2018, 24, 487